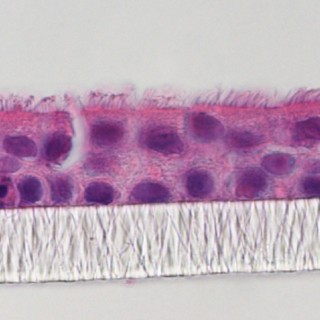
AIRWAY EPITHELIAL CELL CULTURE
Respiratory disorders, whether acute or chronic, communicable, or noncommunicable, affect millions of people and impose a major global burden on health care system. Respiratory diseases are driven by complex interactions within the host-environment and are presented by structural and functional abnormalities. The lack of reproducible in vitro models that closely reflect in vivo physiology hinders new therapeutic approaches for these diseases.
CELLnTEC OFFERS SPECIALTY MEDIA DESIGNED FOR LARGE AIRWAY EPITHELIAL CELLS.
Our class-leading culture medium has been used for expansion of airway, alveolar and bronchial epithelial cells.
The 3D air–liquid interface (ALI) is a key culture condition to develop and differentiate airway epithelial cells in vitro. 3D ALI models of bronchial cells, upper and lower airway, nasal epithelial cells, and 3D culture of lung epithelial cells are achieved with our chemically defined differentiation medium.