AIRWAY EPITHELIAL CELL CULTURE
Infectious diseases rank among the top respiratory system illnesses. Influenza and novel coronavirus-associated pandemics including SARS (2003), MERS (2015), and COVID-19 (2019) have drawn attention to viral infections. Since the identification of SARS-CoV-2 as the causative agent of COVID-19, a worldwide endeavour to study and combat this new health threat is under way.
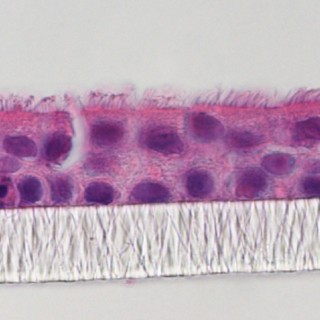
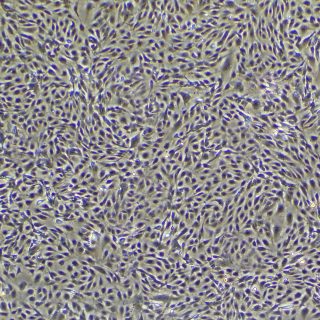
In vitro models using primary cultures of airway epithelial cells have become pivotal tools for research of the pathogenesis of respiratory viruses. Air-liquid-interface (ALI) models epitomize in vivo phenotypes of polarization, beating cilia, barrier, and virus-host cell interactions. 3D ALI models serve as pragmatic instruments for vaccine development and identification of antiviral drug candidates.
CELLnTEC OFFERS SPECIALTY MEDIA DESIGNED FOR LARGE AIRWAY EPITHELIAL CELLS.
CELLnTEC’s market leading precision media have been widely implemented in the development of airway models for studying respiratory viruses like:
- Rhinovirus
- Influenza virus
- Infectious bronchitis virus
- Respiratory syncytial virus