Contains the media and inserts needed to establish 3D epidermal models with improved differentiation and barrier function in your own lab.
| Catalog |
PR3D-K-50 |
| Content |
CnT-PR & CnT-PR-3D media, 48 x Cell culture inserts |
|
|
HIGHLIGHTS:
- Kit contains precision media specially formulated for both proliferation and 3D differentiation
- Kits contains everything needed (except primary cells) to create epidermal models with high barreir functins
- All kit components are fully compatible, ensuring reliable cell behaviour and modelling accuracy
- Cost effective and convenient kit, contains all required products
Description
3D keratinocyte starter Kit was developed for the establishment of 3-dimensional keratinocyte cultures with improved barrier function. Kit includes CnT-Prime medium (CnT-PR) for monolayer culture, CnT-Prime 3D airlift medium (CnT-PR-3D), and 48 cell culture inserts (0.4 um pore size). Kit does not contain primary keratinocytes. Optional extra: CnT-ST-100 stain solution to evaluate confluency prior to airlift.
Back to top
Specifications
- Volume
- 500 ml media (2x)
- Component(s)
- CnT-PR & CnT-PR-3D media, 48 x Cell culture inserts
Back to top
Scientific resources
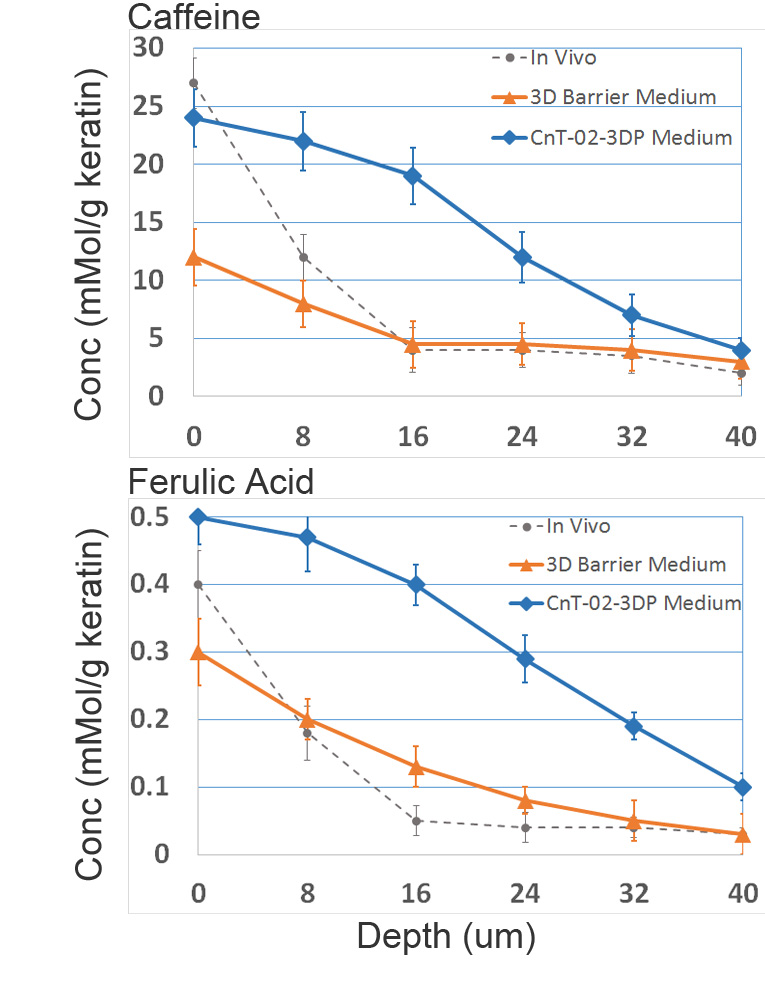
Improved barrier as seen by raman spectroscopy penetration profiles.
The 3D Starter kit without primary keratinocytes (PR3D-K-50) contains the media and inserts needed to establish 3D epidermal models with improved differentiation and barrier function in your own lab.
Early passage human keratinocytes growing in CnT-PR medium are also required.
The kit contains CnT-PR proliferation medium, CnT-PR-3D barrier medium, and 48 inserts for air-lift culture. For details, please see the datasheet.
The 3D models can be routinely established using our recommended protocol.
Following this protocol, the 3D models deliver up to a 50% better barrier function than traditional media. More detailed raman spectroscopy results can be found in the following link:
Improved Barrier Function with 3D Barrier Medium
Back to top
Scientific Literature
| Title |
Year |
| CELLnTEC Catalog |
2023 |
Download |
Back to top


